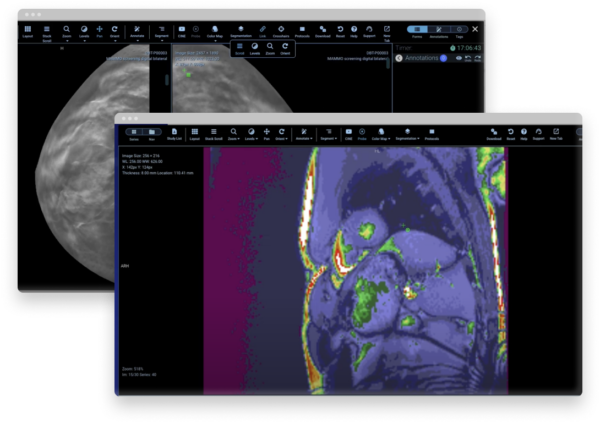
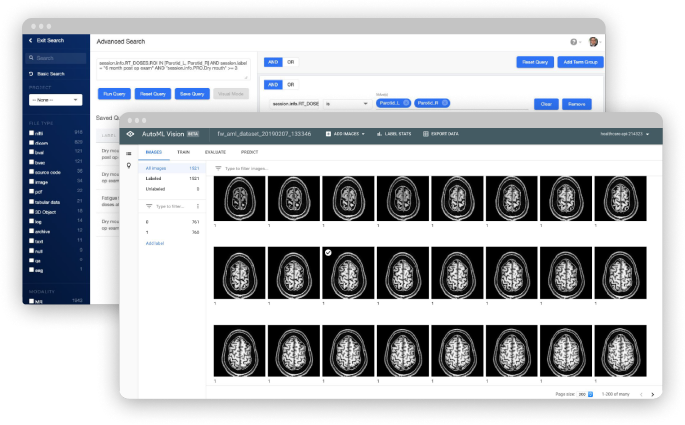
Transform your data curation, analysis, and collaboration
Flywheel Core streamlines imaging research with a medical imaging platform that automates time-consuming processes and scales for your needs. Our secure data management and AI platform gives you the power to create analysis-ready datasets for algorithm training and development.
Schedule A Demo